|
|
| (58 intermediate revisions by the same user not shown) |
| Line 1: |
Line 1: |
| '''PROJECT IDEA''' | | == ''' Physarum Polycephalum.''' == |
|
| |
|
| The original idea was to see if there was any relation between Alii Vibrio fischeri (a specimen that life in warm around 20°C oceans) & Physarum Polycephalum (a specimen that life's in the forest in warm temperatures 20°C). In order to test this, I made an investigation around the two specimens. The second step was to cultivate both of them, but the extraction of the first specimen from an octopus didn't give results, so I redirected my project into teaching Physarum Polycephalum to adapt to salinity environments.
| | '''Project idea.''' |
|
| |
|
| | The original idea was to see if there was any relation between Alii Vibrio fischeri (a specimen that life in warm around 20°C oceans) & Physarum Polycephalum (a specimen that life's in the forest in warm temperatures 20°C). In order to test this, I made an investigation around the two specimens. The second step was to cultivate both of them, sadly the extraction of the first specimen from an octopus didn't give results, so I redirected my project into teaching Physarum Polycephalum to adapt to salinity environments. |
|
| |
|
| | [[File:52h salinity medium.mp4|1500px]] |
|
| |
|
| '''INVESTIGATION OF THE SPECIMENS'''
| | Here can be seen a video around how specimens that were teached to live in different salinity mediums behave when they have the possibility to move between different salinity mediums (Between 0% salinity and 0.5% salinity). It is a 52 h photage of Physarum Polycephalum in salinity medium. |
|
| |
|
| ''' ''Polycephalum/physarum'' '''
| |
|
| |
|
| - Wikipedia link: https://en.wikipedia.org/wiki/Physarum_polycephalum ''(general information around this specimen)''
| | '''Investigation around the specimens.''' |
|
| |
|
| - TED: https://www.ted.com/speakers/heather_barnett_1 ''(a conference opening a window around experiments with this specimen)''
| | In the following links can be found the investigation around the two specimens, the medium that they need and more. |
|
| |
|
| - The Slime Mould Collective: http://slimoco.ning.com/
| | [[/Investigation around Aliivibrio Fischeri.]] |
|
| |
|
| (here can be found experiments around the world around this specimen)
| | [[/Investigation around Physarum Polycephalum.]] |
| ''really cool :) (personal opinion)''
| |
|
| |
|
| - Video ''educational video'' https://www.youtube.com/watch?v=40f7_93NIgA
| |
| ''(Simon Garnier - assistant professor in NJIT talks about his studies)''
| |
|
| |
|
| - Slime Molds Remember - but Do they learn? https://www.quantamagazine.org/slime-molds-remember-but-do-they-learn-20180709/?fbclid=IwAR193K1ULzbxSq-BNUijJWsydeAbAHa6-Eq2sXZpdbLl9_ssvLel3Aph_GM '' " Most importantly, slime molds can be taught new tricks; depending on the species, they may not like caffeine, salt or strong light, but they can learn that no-go areas marked with these are not as bad as they seem, a process known as habituation." '' (This quote from this article, give me a hint, in order to experiment with salinity in the medium of the Polycephalum). Dussutour make an experiment placing oatmeal at the other side of a gelatine substance made with either caffeine or quinine in order to obligate the Polycephalum to cross, she learned that after the organism get used to a certain substance, they will cross to the food ''in this case in the same kind of medium that was located in the first time. (the Physarum have "memory" channels). She made also an experiment testing salinity environments, was the specimen adapted himself to this environment, (also she tested with her team if this information could be communicated to other samples that were not "trained" to this kind of environment).''
| | '''Specimens Mediums.''' |
|
| |
|
| - Video testing intelligence of the specimen https://www.youtube.com/watch?v=czk4xgdhdY4
| | - Medium A.F: |
| | |
| - Video Polycephalum in technology (computer storage) https://www.youtube.com/watch?v=HKZ2LtfDrmg
| |
| | |
| - This specimen is found in the forest and is a type of amoeba.
| |
| | |
| - Grows at a temperature of around 22° - 24°.
| |
| | |
| - Medium: | |
| *100ml of distilled water
| |
| *2g of agar
| |
| *Leaflets
| |
| | |
| | |
| ''' ''Aliivibrio Fischeri'' '''
| |
| | |
| - Wikipedia link:
| |
| | |
| - This specimen is found globally in marine environments.
| |
| | |
| - Is found in very low quantities in almost all oceans of the world in temperate or subtropical water, lives as a symbiont in the light organ of certain fish and squid species. Grows at temperatures of 24°.
| |
| | |
| - The bioluminescence of A. fischeri is caused by transcription of the lux operon, which is induced through population-dependent quorum sensing. The population of this specimen needs to reach an optimal level to activate the lux operon and stimulate light production.
| |
| | |
| - Many people have work with this specimen trying to make their own version of the medium. Common ingredients in most media include Tryptone, yeast extract, glycerol, and roughly 3% NaCl by weight.
| |
| | |
| - Medium:
| |
| *NaCl 30 g | | *NaCl 30 g |
| *Glycerol 1 g | | *Glycerol 1 g |
| Line 57: |
Line 29: |
|
| |
|
|
| |
|
| | - Medium P.P: |
| | *100ml of distilled water |
| | *2g of agar |
| | *Leaflets |
|
| |
|
| '''EXPERIMENTATION with Alii Vibrio Fischeri'''
| |
|
| |
| * Day 1: The extraction of ''Alii Vibrio Fischeri'' from a living natural environment ''(took from an octopus)''. The process consists in taking a sample from the living specimen where the shining in the dark can be seen and then taking a piece of the meet where this specimen lives and putting them all inside of the medium. The sample has to be in a place with 20°C of temperature and in darkness.
| |
|
| |
| [[File:wd11.JPG|400px]]
| |
|
| |
|
| |
| * Day 4: I failed to extract the ''Alii Vibrio Fischeri'' into the medium, after three days there was no blue glowing as seen in the first day inside the medium that I made. My hypothesis is that the specimen wasn't in the same original conditions from where it comes from, and it couldn´t survive.
| |
|
| |
|
| |
| CONCLUSIONS: Because I failed in the extraction of the Aliivibrio Fischeri, I will start with the cultivation of ''Polycephalum/physarum'' in order to confirm if this specimen can be cultivated in salty environments, and more specifically if it can survive in a 3% concentration of NaCl medium. According to the previous investigations of Dussutour, it is possible for it to survive in salinity environments, so I will explore this idea.
| |
|
| |
|
| |
| '''EXPERIMENTATION with ''Polycephalum/physarum'' '''
| |
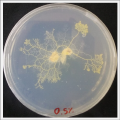
| * Day 1: In order to test if this specimen can grow in salty environments I made the following sample. In the right the two first specimens of ''Polycephalum/physarum'' are in the medium with; 0.5 gr Agar, 25ml water and 1 flake Leaflets. In the middle the medium is divided in the half, the right half has the previous medium and in the left, the medium is made with; 3% of NaCl ''(simulating the salinity of the sea)'', 0.5 gr Agar, 25ml water and 1 flake Leaflet is situated 1mm inside the salty environment. The left sample is made totally with the previous medium ''(the salty medium simulating the salinity of the sea)''.
| |
|
| |
| [[File:wd12.JPG|400px]]
| |
|
| |
| After 5 days the samples of ''Polycephalum/physarum'' didn't came to live again, my first hypothesis is that the medium was to salty my second is that the specimen needs more time in order to come to live again (awake from hibernation state).
| |
|
| |
| [[File:wd23.jpg|400px]]
| |
|
| |
| * Day 2: I made a new sample with a new medium. Parallel to the samples that I use for the experiment I started to use this new Medium to grow the specimen (to have live healthy samples to experiment), I started from the formula that Fernanda Caicedo gets to in her previous experiments, and I altered for my special use.
| |
|
| |
| Medium:
| |
| *100 ml of water
| |
| *4 gr of cooked potatoes
| |
| *4 gr of oat flakes
| |
| *(mix together)
| |
| *2 gr of agar
| |
| *(mix all together and boil)
| |
|
| |
| At left a normal medium, at the center a sample half with the normal medium and the other half with 3% of NaCl, and the third sample with just the salty sample.
| |
|
| |
| [[File:pp-1.jpeg|400px]]
| |
|
| |
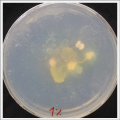
| * Day 3: After one night, the sample in the normal environment is growing normally, the sample in the half mediums moved to the non-salty medium and the one in the salty medium seems that have died.
| |
|
| |
| [[File:pp-2.jpg|400px]]
| |
|
| |
| *Day 7: After 10 days the specimen in the normal conditions environment is trying to find new food, is moving because of some contamination inside the Petri dish, the sample with the half environments didn't growth well, and it seems to be some kind of mutation/contamination/reaction of red color, this event also happened with the samples that I'm growing in order to use in these experiments. The specimens in the salty medium died, but one of the samples have the red color.
| |
|
| |
| [[File:wd24.jpg|400px]]
| |
|
| |
| NOTE: Samples with the pink/red contamination/mutation/reaction. Other samples that I'm growing using the same made medium and the same original Petri dish with the specimen don't have this situation. I don't have an explanation for this fenomena in the moment.
| |
|
| |
|
| [[File:wd25.jpg|400px]]
| |
|
| |
|
| [[File:wd26.jpg|400px]]
| | '''Experimentation with the specimens.''' |
|
| |
|
| *Day 8: A made some new medium ''(this time for the salinity medium I just used 2% of salinity concentration)'' and made a new experiment, this time in the divided medium I used just agar at the left and the salinity 2% concentration medium at left. Two control samples at left with the ideal medium for the growth of the specimen and at the right full concentration of salinity in all the Petri dish.
| | In the following links can be found the diary of work made with each specimen, the experiments that were made with each of them, the experimentation with some mediums and more. |
|
| |
|
| [[File:wd27.jpg|400px]] | | [[/Diary of work: Alii Vibrio Fischeri]] |
|
| |
|
| After one night the specimen in the ideal medium is already growing, the specimen in the middle medium is not moving like the last time ''(probably because this time the food is concentrated in the salinity medium)'' and the samples in the salinity medium seem to be dying again.
| | [[/Diary of work: Physarum Polycephalum]] |
|
| |
|
| [[File:wd28.jpg|400px]]
| |
|
| |
|
| | '''Conclusions:''' |
|
| |
|
| * Day 10: The living samples are turning pink again, and the samples in the salinity medium seem to be like they are dying again.
| | After making the salinity experiment with the Physarum Polycephalum, I get into the conclusion that this specimen can learn to survive in mediums that have salt concentrations. It can learn to survive in them in a process of increasing the salinity, but not if it is situated in a salinity medium without previous teaching. |
|
| |
|
| [[File:wd35.jpg|400px]]
| | After the experiment comes to a lot of questions that can be solved with further experiments like; 1) how fast does it forget? 2) Does it remember after a period of sleep? 3) Is the sclerotium cultivation possible after a period of time?, 4) the specimen showed troubles to find the food in high levels of salinity, Does the levels of NaCl affect the ability of the specimen to find the food? |
| | |
| *Day 12: Definetly the samples in the salinity medium are death. Ant the contamination/chemical reaction continues in the other samples.
| |
| | |
| [[File:wd36.jpg|400px]]
| |
| | |
| * Day 15: Because the salinity in the medium is to high for the specimens to growth, I reduced the salinity inside the medium into .3% in order to see the reaction of the specimen. The right samples continues to be just a control sample with an ideal medium and the medium in the middle is a medium in between with food in the none salinity medium.
| |
| | |
| [[File:wd37.jpg|400px]]
| |
| | |
| * Day 17: For the ideal medium the specimen is growing, but after a week it acquire some mode, the middle sample move to the food area away from the salinity medium, and the specimen in the salinity medium started to move in order to try to find food. It is tolerating the salinity medium. The specimen in the salinity medium with 0.3% concentration of NaCl is starting to get into hibernation mode, I put some food in order to make it strong before transplanting it into a new medium with higher concentrations of NaCl.
| |
| | |
| [[File:wd38.jpg|400px]]
| |
| | |
| *Day 19: I made an observation under the microscope, the pink color in my samples is a bacteria, contamination that comes from the first petri dish that I used to extract a clean sample. Here can be found more pictures of the contamination.
| |
| Because of health conditions and the use that can be given in the laboratory where I work, I put away this samples (disposed of them in a clean way).
| |
|
| |
|
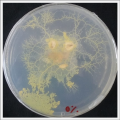
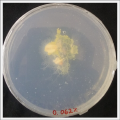
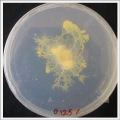
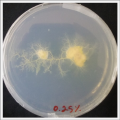
| | ''Salinity Percentage Mediums: ''Strains P.p.P'' |
| <gallery> | | <gallery> |
| File:wd40.jpg | | File:0% salinity.png |
| File:wd41.jpg | | File:0.062% salinity.png |
| File:wd42.jpg | | File:0.125% salinity.png |
| File:wd43.jpg | | File:0.25% salinity.png |
| File:wd45.jpg | | File:0.5% salinity.png |
| | File:1% salinity.png |
| </gallery> | | </gallery> |
|
| |
|
| | During the time that I adopted this specimen, I made many tests in order to get to know and understand the specimen. I'm also taking the class of "DIY Bio; doing things with biology", where I made many tests in terms of food, temperature, and light. |
|
| |
|
| *Day 20: I continue increasing the salinity quantities in the medium of the specimen to 0.4% and 0.5%. It is working.
| | This information can be found on the following website along with information of other experimentation: |
| Because of the vacation period, I put all my samples into rest (hibernation) and I will wake them up again in 3 weeks.
| |
| | |
| [[File:wd47.jpg|400px]]
| |
| [[File:wd48.jpg|400px]]
| |
| | |
| *Day 45:
| |
| I try without success to revive my trained samples of Physarum polycephalum. My hypothesis is that the medium where I tried to revive them was not the one with the salinity concentration where they were taught before that they will find food. Sadly I don't have more samples of the taught samples in .04Nacl to prove my theory.
| |
| | |
| *Day 53:
| |
| I cultivate new samples of the specimen in ordere to start the test of adaptation again.
| |
| | |
| | |
| | |
| | |
| '''CONCLUSIONS:'''
| |
| | |
| | |
| | |
| Datasheet: Physarum Polycephalum
| |
|
| |
|
| [[:File: Physarum Polycephalum.pdf]]
| | - https://www.uni-weimar.de/kunst-und-gestaltung/wiki/GMU:DIY_Bio:_doing_things_with_biology/Paola_Stephania_Calder%C3%B3n |