No edit summary |
|||
| (31 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Object is talking == | |||
== | ===1. Introduction=== | ||
1.1 anthocyanin | 1.1 anthocyanin | ||
Anthocyanins are water-soluble vacuolar pigments. In the 1664 book Experiments and Considerations Touching Colours by chemist Robert Boyle, various edible plants are reported as visual pH indicators due to pH-responsive mechanisms in their tissues [1]. Anthocyanin is a kind of natural colorant in food and beverage industry, and has been found to possess anti-inflammatory antioxidant properties [2]. It has also been researched for use as an indicator for packaging applications to detect spoilage in pork and fish products [3]. All tissues of vascular plants contain the flavonoid anthocyanin, a pigment that changes colour under varying pH solutions. | Anthocyanins are water-soluble vacuolar pigments. In the 1664 book Experiments and Considerations Touching Colours by chemist Robert Boyle, various edible plants are reported as visual pH indicators due to pH-responsive mechanisms in their tissues [1]. Anthocyanin is a kind of natural colorant in food and beverage industry, and has been found to possess anti-inflammatory antioxidant properties [2]. It has also been researched for use as an indicator for packaging applications to detect spoilage in pork and fish products [3]. All tissues of vascular plants contain the flavonoid anthocyanin, a pigment that changes colour under varying pH solutions. | ||
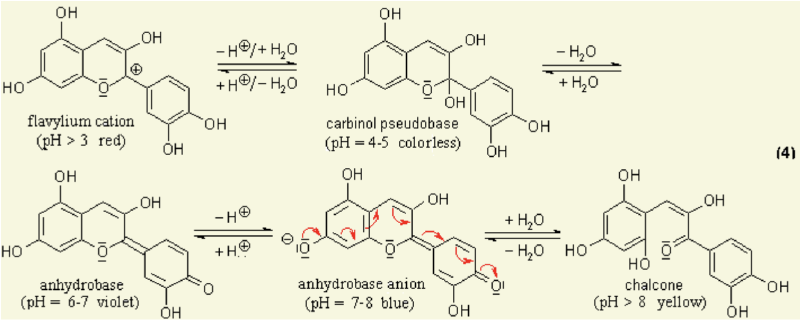
Under different pH conditions, the hydroxyl (OH) and/or methyl ether (O-CH3) groups attached to the carbon rings (figure 1) undergo reversible structural transformations and ionizations. Restructuring a molecule changes the way it ab- sorbs light, giving rise to colour changes [4]. | Under different pH conditions, the hydroxyl (OH) and/or methyl ether (O-CH3) groups attached to the carbon rings (figure 1) undergo reversible structural transformations and ionizations. Restructuring a molecule changes the way it ab- sorbs light, giving rise to colour changes [4]. | ||
[[File:Screen Shot 2018-01-31 at 12.08.56.png| | [[File:Screen Shot 2018-01-31 at 12.08.56.png|800px]] | ||
Chemical diagram of colour-changing anthocyanin pH reaction [5] | |||
1.2 red cabbage | 1.2 red cabbage | ||
"Red cabbage is rich in a number of bioactive substances, including anthocycanins"[Wiczkowski, 2012]. The method of extracting anthocyanin from red cabbage is easy and convenient. | |||
===2. Idea=== | |||
This work is based on a research done by MIT media lab that uses organic fluid-based molecules. The molecules anthocyanin, vanillin, chitosan are used as dopants that can sense different pH values. The output is in the form of a broad spectrum of colors, odors and shapes. Based on this, the experiment focus on color changing, using kappa-carrageenan as substrate to present the reaction in a certain form. The outcome shows chemical reactions that are in daily life unreadable and unseen. What is shown here is a new design language to create new appliances. In the exhibition, color changing can be seen both in liquid and solid statues. | |||
===3. Method=== | |||
<gallery caption="Color Changing in liquid status"> | |||
File:4931517416494_.pic_hd.jpg|Making red cabbage indicator | |||
File:Screen Shot 2018-01-31 at 15.12.21.png|Making pH solutions | |||
</gallery> | |||
<gallery caption="Color Changing in solid status"> | |||
File:屏幕快照 2018-03-31 下午1.40.34.png|1.5%w/v kappa-carrageenan in deionized water | |||
File:屏幕快照 2018-03-31 下午1.41.12.png|pour anthocyanin solution | |||
File:屏幕快照 2018-03-31 下午1.41.26.png|make a color film | |||
File:屏幕快照 2018-03-31 下午1.41.39.png|dry the color film | |||
File:屏幕快照 2018-03-31 下午1.42.22.png|color changing | |||
File:屏幕快照 2018-03-31 下午1.42.02.png|color changing | |||
</gallery> | |||
<gallery caption="Shape Changing"> | |||
File:2018-03-31 下午1.42.36.png|4% w/v chitosan powder | |||
File:屏幕快照 2018-03-31 下午3.21.01.png|3% v/v acetic acid | |||
File:屏幕快照 2018-03-31 下午1.42.48.png|pour chitisan solution | |||
File:屏幕快照 2018-03-31 下午1.43.01.png|make a shape film | |||
File:屏幕快照 2018-03-31 下午1.43.20.png|dry the shape film | |||
File:屏幕快照 2018-03-31 下午1.43.34.png|0.75 uL droplets of the pH solution drop on the shape film | |||
File:屏幕快照 2018-03-31 下午1.43.45.png|pH2 and pH12 shape changing | |||
</gallery> | |||
{{#evt:service=vimeo|id=262637645}} | |||
<br/> | |||
1. | |||
-- | |||
- | |||
[[ | ===References=== | ||
#[1] Robert Boyle. 1664. Experiments and Considerations Touching Colours. Project Gutenberg. 1–157 pages. | |||
#[2] Jian He and M Monica Giusti. 2010. Anthocyanins: Natural Colorants with Health-Promoting Properties. | |||
#[3] Xiahong Zhang, Sisi Lu, and Xi Chen. 2014. A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn. Sensors and Actuators B: Chemical 198 (2014), 268–273. | |||
#[4] Viirj Kan. 2017. Organic Primitives: Synthesis and Design of pH-Reactive Materials using Molecular I/O for Sensing, Actuation, and Interaction. Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems Pages 989-1000. | |||
#[5] UMass Amherst Department of Chemistry Lecture Demonstrations. | |||
#[6] https://ellieirons.com/projects/two-meadows/ | |||
[[:File:12.6 bio-lab first idea.pdf]] | |||
Latest revision as of 10:29, 5 April 2018
Object is talking
1. Introduction
1.1 anthocyanin Anthocyanins are water-soluble vacuolar pigments. In the 1664 book Experiments and Considerations Touching Colours by chemist Robert Boyle, various edible plants are reported as visual pH indicators due to pH-responsive mechanisms in their tissues [1]. Anthocyanin is a kind of natural colorant in food and beverage industry, and has been found to possess anti-inflammatory antioxidant properties [2]. It has also been researched for use as an indicator for packaging applications to detect spoilage in pork and fish products [3]. All tissues of vascular plants contain the flavonoid anthocyanin, a pigment that changes colour under varying pH solutions. Under different pH conditions, the hydroxyl (OH) and/or methyl ether (O-CH3) groups attached to the carbon rings (figure 1) undergo reversible structural transformations and ionizations. Restructuring a molecule changes the way it ab- sorbs light, giving rise to colour changes [4].
Chemical diagram of colour-changing anthocyanin pH reaction [5]
1.2 red cabbage "Red cabbage is rich in a number of bioactive substances, including anthocycanins"[Wiczkowski, 2012]. The method of extracting anthocyanin from red cabbage is easy and convenient.
2. Idea
This work is based on a research done by MIT media lab that uses organic fluid-based molecules. The molecules anthocyanin, vanillin, chitosan are used as dopants that can sense different pH values. The output is in the form of a broad spectrum of colors, odors and shapes. Based on this, the experiment focus on color changing, using kappa-carrageenan as substrate to present the reaction in a certain form. The outcome shows chemical reactions that are in daily life unreadable and unseen. What is shown here is a new design language to create new appliances. In the exhibition, color changing can be seen both in liquid and solid statues.
3. Method
- Color Changing in liquid status
- Color Changing in solid status
- Shape Changing
References
- [1] Robert Boyle. 1664. Experiments and Considerations Touching Colours. Project Gutenberg. 1–157 pages.
- [2] Jian He and M Monica Giusti. 2010. Anthocyanins: Natural Colorants with Health-Promoting Properties.
- [3] Xiahong Zhang, Sisi Lu, and Xi Chen. 2014. A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn. Sensors and Actuators B: Chemical 198 (2014), 268–273.
- [4] Viirj Kan. 2017. Organic Primitives: Synthesis and Design of pH-Reactive Materials using Molecular I/O for Sensing, Actuation, and Interaction. Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems Pages 989-1000.
- [5] UMass Amherst Department of Chemistry Lecture Demonstrations.
- [6] https://ellieirons.com/projects/two-meadows/
File:12.6 bio-lab first idea.pdf