Jan Glöckner (talk | contribs) No edit summary |
No edit summary |
||
| (30 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[/The digital lab-book|The digital lab-book]] ( this is the unedited version of the lab-book, that will be the most up-to-date document) | |||
[[/General methods|General methods]] | |||
==supermarket piracy programm== | |||
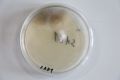
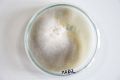
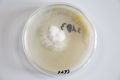
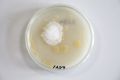
Oyster mushroom cloning series stage 1 (obtained from Globus supermarket; polish origin) | |||
<gallery> | |||
File:Pleur.ostr.SAD1.JPG| | |||
File:Pleur.ostr.SAD1 alternateview.JPG| | |||
File:Pleur.ostr.SAD2.JPG| | |||
File:Pleur.ostr.SAD3.JPG| | |||
File:Pleur.ostr.SAD4.JPG| | |||
File:Pleur.ostr.SBD1.JPG| | |||
File:Pleur.ostr.SBD2.JPG| | |||
File:Pleur.ostr.SBD3.JPG| | |||
File:Pleur.ostr.SBD3 alternateview.JPG| | |||
</gallery> | |||
Brown Button mushroom cloning series stage 1,2 and stage 3 [petri dishes A to D and G.H not shown] ( obtained from Rewe supermarket; german origin) | |||
<gallery> | |||
File:button E1 alternateview.JPG | |||
File:button E2.JPG | |||
File:button E3.JPG | |||
</gallery> | |||
===Artistic Abstract=== | |||
To address the growing issue of patented living organisms, I pirate the genome of store-bought mushroom-products, by reverse engineering the product to its viable state of mycelium. I then distribute the organism, for everyone to culture and distribute, in a respectful way and to form mutual diplomatic relations. As the genetic makeup of the strains change through cultivation, the creative commons licence is applied to the genome to keep it from being obscured by companies. | |||
[https://creativecommons.org/licenses/by-sa/4.0/ CC BY-SA 4.0] | |||
===Scientific Abstract=== | |||
Specimen of mushrooms obtained from supermarkets are cloned and the developing strain is purified, than stored in a cold-storage strain-bank. Via reverse-engineering the strain from a commercial product is extracted and cultured. The strains will be given away, and kept available. | |||
===Method=== | |||
For every attempt of cloning at least seven petri-dishes filled with PDA- medium are inoculated. The scalpel is sterilized in a hot flame till it glows red. It then is cooled in the receiving petri dish. The mushroom is ripped apart in the middle. Tissue then is taken from just above the gills, inside the stem or just under the cap. It is inserted in the hole that the hot scalpel made. The dishes are labeled and incubated in the incubator (23°-25° C). The mycelium that emerges is purified by running it through subsequent petri dishes till there is no visible evidence of contaminants. Then it is stored in test-tubes filled with PDA- medium at temperatures below 5°C. Two tubes per strain, enveloped in a zip-loc-bag. ( liquid suspended-animation technique will be introduced.) | |||
===References=== | |||
*Paul Stamets and J.S. Chilton: The Mushroom Cultivator; Sterile Technique and Agar Culture: Starting a Culture From Live Tissue (p. 29) 1983 Agarikon Press Olympia Washington ISBN: 978-0-9610798-0-2 | |||
*Paul Stamets: Growing Gourmet and Medicinal Mushrooms (Third Edition); Culturing Mushroom Mycelium on Agar Media: Starting a Mushroom Strain by Cloning (p. 88); 2000 Ten Speed Press ( Random House New York) ISBN: 978-1-58008-175-7 | |||
Latest revision as of 15:56, 31 March 2018
The digital lab-book ( this is the unedited version of the lab-book, that will be the most up-to-date document)
supermarket piracy programm
Oyster mushroom cloning series stage 1 (obtained from Globus supermarket; polish origin)
Brown Button mushroom cloning series stage 1,2 and stage 3 [petri dishes A to D and G.H not shown] ( obtained from Rewe supermarket; german origin)
Artistic Abstract
To address the growing issue of patented living organisms, I pirate the genome of store-bought mushroom-products, by reverse engineering the product to its viable state of mycelium. I then distribute the organism, for everyone to culture and distribute, in a respectful way and to form mutual diplomatic relations. As the genetic makeup of the strains change through cultivation, the creative commons licence is applied to the genome to keep it from being obscured by companies.
Scientific Abstract
Specimen of mushrooms obtained from supermarkets are cloned and the developing strain is purified, than stored in a cold-storage strain-bank. Via reverse-engineering the strain from a commercial product is extracted and cultured. The strains will be given away, and kept available.
Method
For every attempt of cloning at least seven petri-dishes filled with PDA- medium are inoculated. The scalpel is sterilized in a hot flame till it glows red. It then is cooled in the receiving petri dish. The mushroom is ripped apart in the middle. Tissue then is taken from just above the gills, inside the stem or just under the cap. It is inserted in the hole that the hot scalpel made. The dishes are labeled and incubated in the incubator (23°-25° C). The mycelium that emerges is purified by running it through subsequent petri dishes till there is no visible evidence of contaminants. Then it is stored in test-tubes filled with PDA- medium at temperatures below 5°C. Two tubes per strain, enveloped in a zip-loc-bag. ( liquid suspended-animation technique will be introduced.)
References
- Paul Stamets and J.S. Chilton: The Mushroom Cultivator; Sterile Technique and Agar Culture: Starting a Culture From Live Tissue (p. 29) 1983 Agarikon Press Olympia Washington ISBN: 978-0-9610798-0-2
- Paul Stamets: Growing Gourmet and Medicinal Mushrooms (Third Edition); Culturing Mushroom Mycelium on Agar Media: Starting a Mushroom Strain by Cloning (p. 88); 2000 Ten Speed Press ( Random House New York) ISBN: 978-1-58008-175-7