Freya Probst (talk | contribs) No edit summary |
Freya Probst (talk | contribs) |
||
| Line 64: | Line 64: | ||
7.5 g Agar | 7.5 g Agar | ||
We had 6 Petridishes that contained our medium and our pipette could collect 3 ml of liquid. As a first step, we blended a piece of the Kombucha skin with 0.5 ml of distilled water and poured a drop onto our first dish. The first 0.5 ml of bacteria-solution was diluted further | |||
We emptied the pipette again till we regained the amount of 0.5 ml and added another 2.5 ml of distilled water. | :We had 6 Petridishes that contained our medium and our pipette could collect 3 ml of liquid. As a first step, we blended a piece of the Kombucha skin with 0.5 ml of distilled water and poured a drop onto our first dish. The first 0.5 ml of bacteria-solution was diluted further with additional 2.5 ml of distilled water. Another drop was poured onto the second dish. We emptied the pipette again till we regained the amount of 0.5 ml and added another 2.5 ml of distilled water. | ||
{| | {| | ||
| Line 82: | Line 82: | ||
|'''1:7776''' | |'''1:7776''' | ||
|- | |- | ||
| [[File:1-216 Gluconacetobacter xylinus.jpg | | [[File:1-216 Gluconacetobacter xylinus.jpg|250 px]] | ||
| [[File:1-1296 Gluconacetobacter xylinus.jpeg|250px]] | | [[File:1-1296 Gluconacetobacter xylinus.jpeg|250px]] | ||
| [[File:1-7776 Gluconacetobacter xylinus.jpg|250px]] | | [[File:1-7776 Gluconacetobacter xylinus.jpg|250px]] | ||
|} | |} | ||
We could observe how the Gluconacetobacter colonies arranged differently according to its concentration on the petridish. | |||
Revision as of 18:37, 18 November 2016
Basic growth medium for bacteria
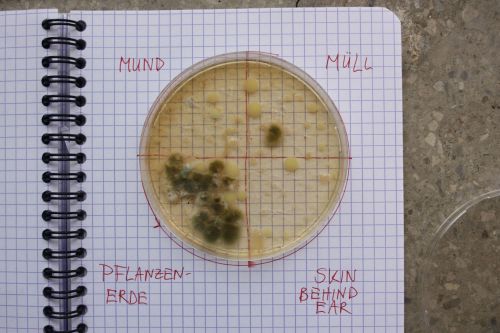
- How can we, as people without access to a professional laboratory, grow microorganisms?
1. Procedure:
- The petri dishes are cleaned with a solution of 70% Alcohol + 30% of water.
200 ml destilled water/ tapwater 3 ts sugar 2 ts bouillon/ peptone (proteins) 2 g agar (gellant)
We mixed different mediums, varying the ingredients. Tapwater and bouillon are easily accessible, while peptone and destilled water are more specific.
Mine contained distilled water, but instead of peptone I used bullion.
2. Outcome:
- Only the soil sample showed some result. Besides that we can only observe oil drops from the Boullion.
Bacteria tasting
- Fermentation is a way of preserving food and the reason for that lies within its sour flavour. Under these sour conditions, neither mushrooms nor bacteria (other than our Lactobacillales) will grow. In our stomachs Lactobacillales takes over important functions and we won't kill it by drinking it. However after taking antibiotics humans can repopulate their gut flora by eating or drinking something probiotic.
1. Milk -> Yoghurt:
kill possibly pathogenic organisms in the milk: 90°C cool milk down to body temperature: 40°C add the Lactobacillales (in a few spoons of yoghurt)
- Inside of the incubator the bacteria eat the lactose found in the milk.
2. Kombucha Tea:
1 cup black/green tea 4 ts (or more) sugar add lent or bought Gluconacetobacter xylinus (after the tea cooled down to room temperature)
Serial dilution of bacteria
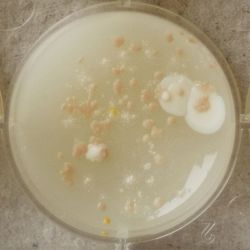
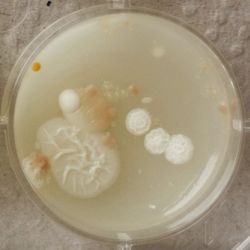
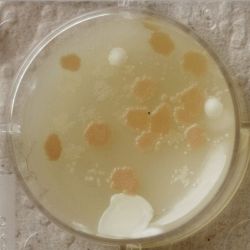
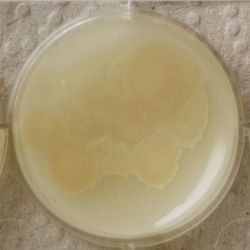
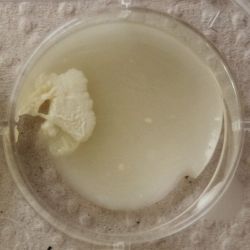
- Under the right conditions one Bacteria can divide itself infinetely and pile up a colony of its kind. The procedure of serial dilution (reducing the amount of bacteria in a solution within steps) is for example useful for counting bacteria. The Gluconacetobacter that we extracted out of the Kombucha Tea also changed its colonies with different concentration of bacteria in a solution (distilled water).
Medium for the Gluconacetobacter:
10 g Glucose 2.5 g Peptone 2.5 g Yeast extract 1.35 g Na2HPO4 0.75 g Citric acid 500 ml Distilled water 7.5 g Agar
- We had 6 Petridishes that contained our medium and our pipette could collect 3 ml of liquid. As a first step, we blended a piece of the Kombucha skin with 0.5 ml of distilled water and poured a drop onto our first dish. The first 0.5 ml of bacteria-solution was diluted further with additional 2.5 ml of distilled water. Another drop was poured onto the second dish. We emptied the pipette again till we regained the amount of 0.5 ml and added another 2.5 ml of distilled water.
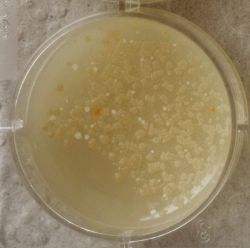
| 1:1 | 1:6 | 1:36 |
|
|
|
| 1:216 | 1:1296 | 1:7776 |
|
|
|
We could observe how the Gluconacetobacter colonies arranged differently according to its concentration on the petridish.