Paola S. Calderón/Project: Specimen ''Physarum Polycephalum''.: Difference between revisions
| Line 18: | Line 18: | ||
[[/Investigation around Physarum Polycephalum.]] | [[/Investigation around Physarum Polycephalum.]] | ||
''' | |||
'''Specimens Experimentation.''' | |||
In the following links can be found the diary of work made with each specimen, the experiments that were made with each of them, the experimentation with some mediums and more. | In the following links can be found the diary of work made with each specimen, the experiments that were made with each of them, the experimentation with some mediums and more. | ||
Revision as of 20:53, 25 March 2019
Physarum Polycephalum.
Project idea.
The original idea was to see if there was any relation between Alii Vibrio fischeri (a specimen that life in warm around 20°C oceans) & Physarum Polycephalum (a specimen that life's in the forest in warm temperatures 20°C). In order to test this, I made an investigation around the two specimens. The second step was to cultivate both of them, sadly the extraction of the first specimen from an octopus didn't give results, so I redirected my project into teaching Physarum Polycephalum to adapt to salinity environments.
Here can be seen a video around how specimens that were teached to live in different salinity mediums behave when they have the possibility to move between different salinity mediums (Between 0% salinity and 0.5% salinity). It is a 52 h photage of Physarum Polycephalum in salinity medium.
Investigation around the specimens.
In the following links can be found the investigation around the two specimens, the medium that they need and more.
/Investigation around Aliivibrio Fischeri.
/Investigation around Physarum Polycephalum.
Specimens Experimentation.
In the following links can be found the diary of work made with each specimen, the experiments that were made with each of them, the experimentation with some mediums and more.
/Diary of work: Alii Vibrio Fischeri
/Diary of work: Physarum Polycephalum
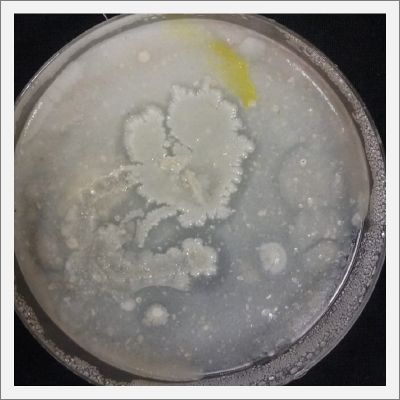
EXPERIMENTATION with Polycephalum/physarum
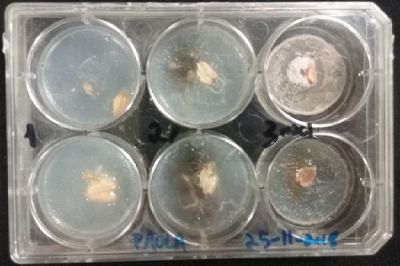
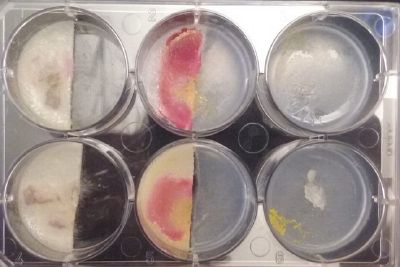
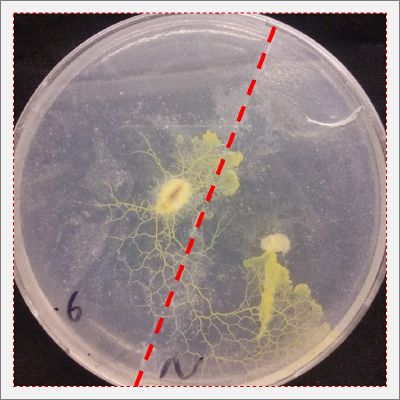
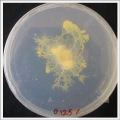
- Day 1: In order to test if this specimen can grow in salty environments I made the following sample. In the right the two first specimens of Polycephalum/physarum are in the medium with; 0.5 gr Agar, 25ml water and 1 flake Leaflets. In the middle the medium is divided in the half, the right half has the previous medium and in the left, the medium is made with; 3% of NaCl (simulating the salinity of the sea), 0.5 gr Agar, 25ml water and 1 flake Leaflet is situated 1mm inside the salty environment. The left sample is made totally with the previous medium (the salty medium simulating the salinity of the sea).
After 5 days the samples of Polycephalum/physarum didn't come to live again, my first hypothesis is that the medium was too salty my second is that the specimen needs more time in order to come to live again (awake from hibernation state).
- Day 2: I made a new sample with a new medium. Parallel to the samples that I use for the experiment I started to use this new Medium to grow the specimen (to have live healthy samples to experiment), I started from the formula that Fernanda Caicedo gets to in her previous experiments, and I altered for my special use.
Medium:
- 100 ml of water
- 4 gr of cooked potatoes
- 4 gr of oat flakes
- (mix together)
- 2 gr of agar
- (mix all together and boil)
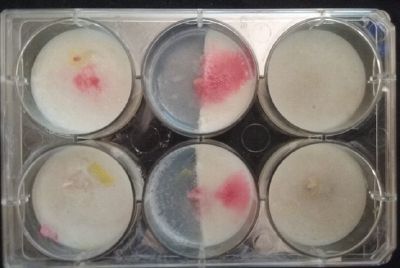
At left a normal medium, at the center a sample half with the normal medium and the other half with 3% of NaCl, and the third sample with just the salty sample.
- Day 3: After one night, the sample in the normal environment is growing normally, the sample in the half mediums moved to the non-salty medium and the one in the salty medium seems that have died.
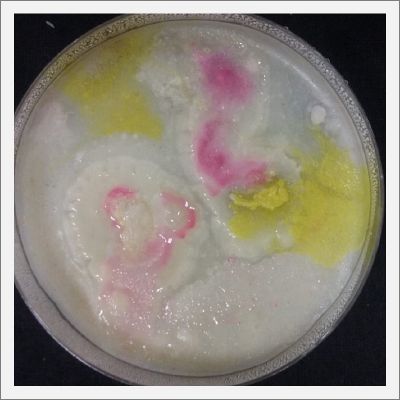
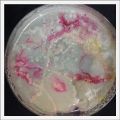
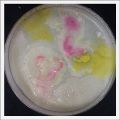
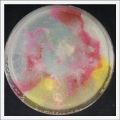
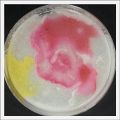
- Day 7: After 10 days the specimen in the normal conditions environment is trying to find new food, is moving because of some contamination inside the Petri dish, the sample with the half environments didn't growth well, and it seems to be some kind of mutation/contamination/reaction of red color, this event also happened with the samples that I'm growing in order to use in these experiments. The specimens in the salty medium died, but one of the samples have a red color.
NOTE: Samples with the pink/red contamination/mutation/reaction. Other samples that I'm growing using the same made medium and the same original Petri dish with the specimen doesn't have this situation. I don't have an explanation for this phenomena at the moment.
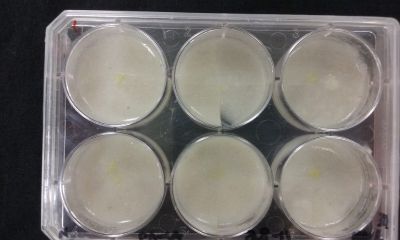
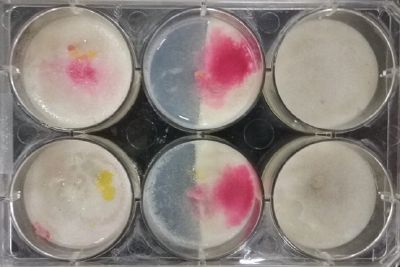
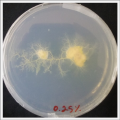
- Day 8: A made some new medium (this time for the salinity medium I just used 0.2% of salinity concentration) and made a new experiment, this time in the divided medium I used just agar at the left and the salinity 0.2% concentration medium at left. Two control samples at left with the ideal medium for the growth of the specimen and at the right full concentration of salinity in all the Petri dish.
After one night the specimen in the ideal medium is already growing, the specimen in the middle medium is not moving like the last time (probably because this time the food is concentrated in the salinity medium) and the samples in the salinity medium seem to be dying again.
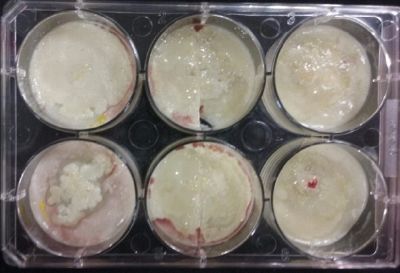
- Day 10: The living samples are turning pink again, and the samples in the salinity medium seem to be like they are dying again.
- Day 12: Definitely the samples in the salinity medium are death. Ant the contamination/chemical reaction continues in the other samples.
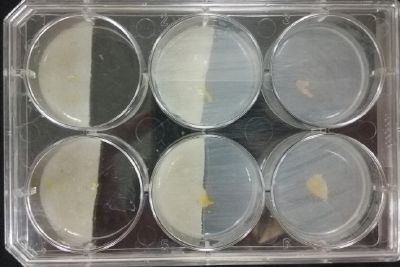
- Day 15: Because the salinity in the medium is too high for the specimens to growth, I reduced the salinity inside the medium into 0.03% in order to see the reaction of the specimen. The right samples continue to be just a control sample with an ideal medium and the medium in the middle is a medium in between with food in the none salinity medium.
- Day 17: For the ideal medium the specimen is growing, but after a week it acquire some mode, the middle sample move to the food area away from the salinity medium, and the specimen in the salinity medium started to move in order to try to find food. It is tolerating the salinity medium. The specimen in the salinity medium with 0.03% concentration of NaCl is starting to get into hibernation mode, I put some food in order to make it strong before transplanting it into a new medium with higher concentrations of NaCl.
- Day 19: I made an observation under the microscope, the pink color in my samples is a bacteria, contamination that comes from the first petri dish that I used to extract a clean sample. Here can be found more pictures of the contamination.
Because of health conditions and the use that can be given in the laboratory where I work, I put away these samples (disposed of them in a clean way).
- Day 20: I continue increasing the salinity quantities in the medium of the specimen to 0.04% and 0.05%. It is working.
Because of the vacation period, I put all my samples into rest (hibernation) and I will wake them up again in 3 weeks.
- Day 45:
I try without success to revive my trained samples of Physarum polycephalum. My hypothesis is that the medium where I tried to revive them was not the one with the salinity concentration where they were taught before that they will find food. Sadly I don't have more samples of the taught samples in 0.04% NaCl to prove my theory.
- Day 53:
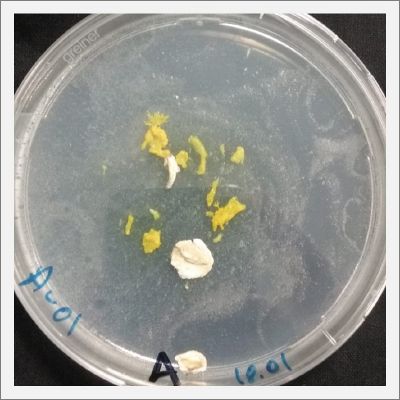
I cultivate new polycephalum taken from samples of Niilo (A-#), Julian (B-#), Antje(C-#), and in order to start the test of adaptation again.
- Day 55:
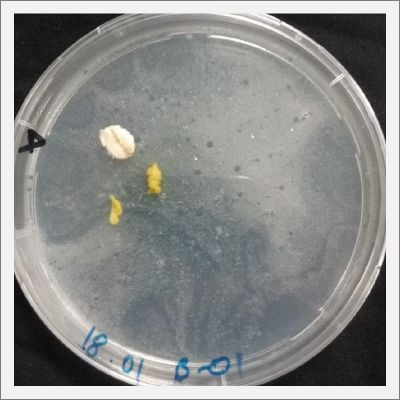
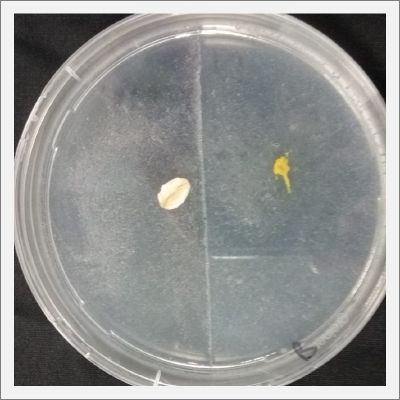
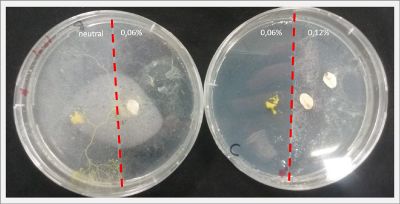
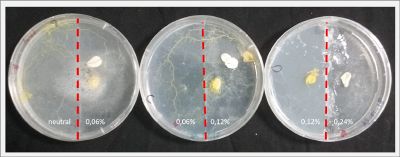
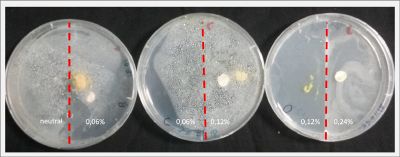
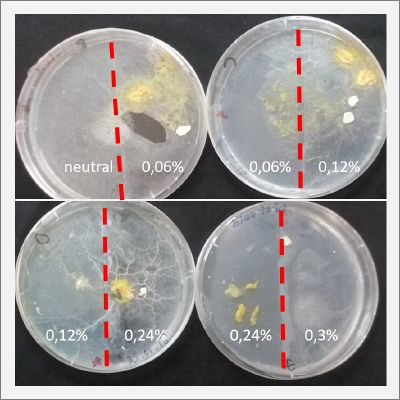
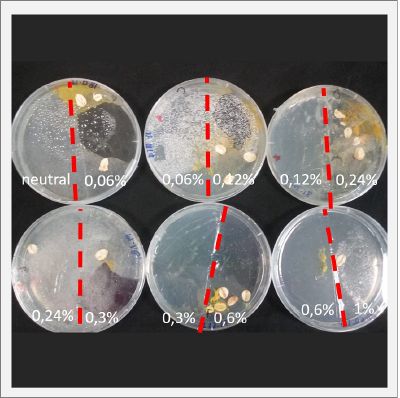
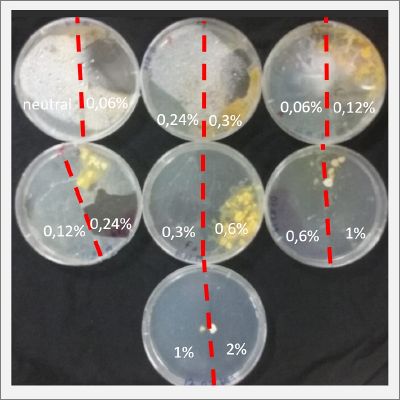
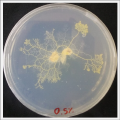
With these new healthy samples of the specimen, I made two Petri dishes in order to reeducate Physarum Policephalum to live in salinity environments. To do that I made a neutral medium (100ml of water + 2 gr of agar) and a medium with 0.06% of salinity (100ml of water + 2 gr of agar + 0.06 gr of NaCl) I put the Pysarum Policephalum in the neutral medium and the food in the salinity medium. I increase the salinity till 0.06% to test if the specimen is able to adapt to this salinity environment according to the results I receive from the first tests.
- Day 57:
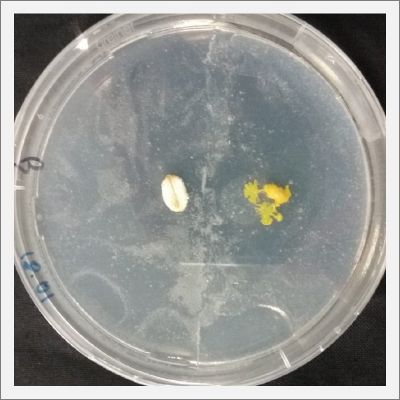
After two days the specimen moved to the salinity 0.06% medium in order to find the food and is tolerating this environment/medium.
- Day 59:
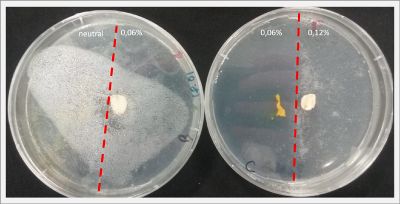
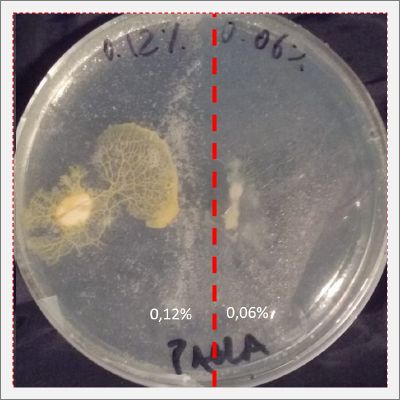
I create new Petri dishes the half is the previous environment with 0.06% of salinity (there I situated the Physarum polycephalum) and the other half is with 0.12% of salinity (there I situated the food).
- Day 61:
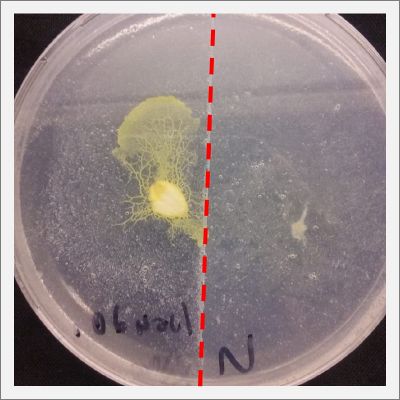
After two days the specimen moved to the salinity 0.12% medium in order to find the food and is tolerating this environment/medium.
- Day 63:
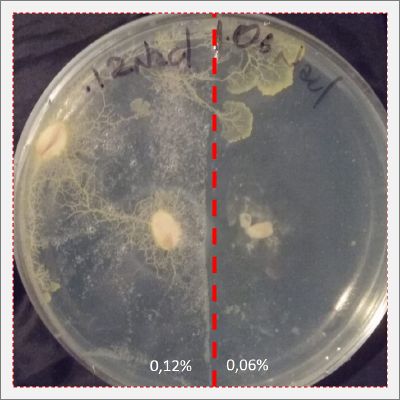
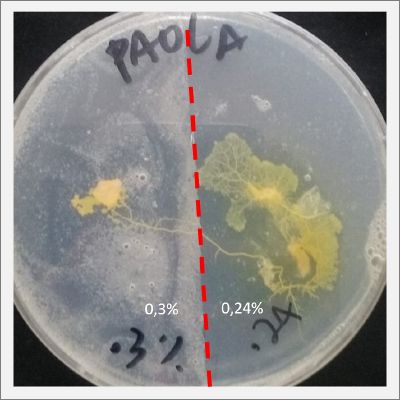
I create new Petri dishes the half is the previous environment with 0.12% of salinity (there I situated the Physarum polycephalum) and the other half is with 0.24% of salinity (there I situated the food).
- Day 65:
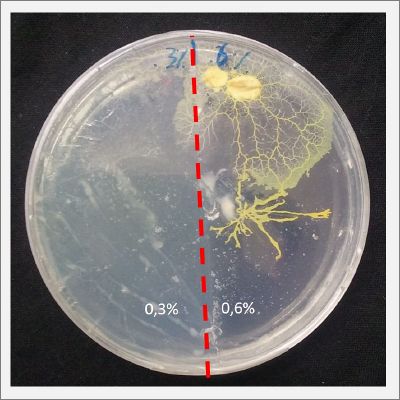
I create new petri dishes the half is the previous environment with 0.24% of salinity (there I situated the Physarum polycephalum) and the other half is with 0.3% of salinity (there I situated the food). One of the previous samples got contamination so I continue with just one sample.
- Day 67:
After two days the specimen moved to the salinity 0.3% medium in order to find the food and is tolerating this environment/medium.
- Day 69:
I create new Petri dishes the half is the previous environment with 0.3% of salinity (there I situated the Physarum polycephalum) and the other half is with 0.6% of salinity (there I situated the food).
- Day 74:
After two days the specimen hasn't moved to the salinity 0.6% medium, I give it 3 more days and after this time the specimen moved to the salinity 0.6% medium in order to find the food and is tolerating this environment/medium.
- Day 75:
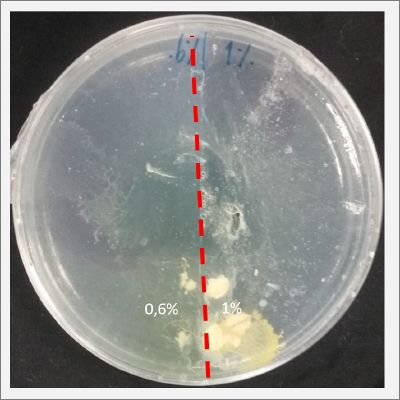
I create new Petri dishes the half is the previous environment with 0.6% of salinity (there I situated the Physarum polycephalum) and the other half is with 1% salinity (there I situated the food).
- Day 76:
After two days the specimen moved to the salinity 1% medium in order to find the food and is tolerating this environment/medium.
- Day80:
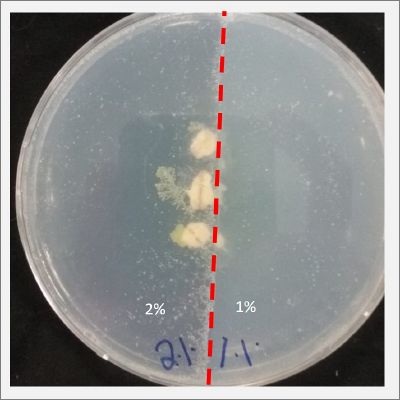
I create new Petri dishes the half is the previous environment with 1% of salinity (there I situated the Physarum polycephalum) and the other half is with 2% salinity (there I situated the food).
- Day 86:
After six days the specimen moved to the salinity 1% medium in order to find the food and is tolerating this environment/medium. The specimen is not growing to fast, so I will let it more time into the new salinity medium, in order for it to adapt.
- Day90:
I create new Petri dishes the half is the previous environment with 2% of salinity (there I situated the Physarum polycephalum) and the other half is with 3% salinity (there I situated the food).
- Day 92:
- Day 100: I translated the specimens into new Petri dishes with their new level of salinity in order to keep the specimens healthy.
Salinity Percentage mediums
CONCLUSIONS:
During the time that I adopted this specimen, I made many tests in order to get to know and understand the specimen. I'm also taking the class of "DIY Bio; doing things with biology", where I made many tests in terms of food, temperature, and light. This information can be found on the following websites:
After making the salinity experiment with the Physarum Polycephalum, I get into the conclusion that this specimen can learn to survive in mediums that have salt concentrations. It can learn to survive in them in a process of increasing the salinity, but not if it is situated in a salinity medium without previous teaching.