No edit summary |
No edit summary |
||
| Line 128: | Line 128: | ||
[[File:wd38.jpg|400px]] | [[File:wd38.jpg|400px]] | ||
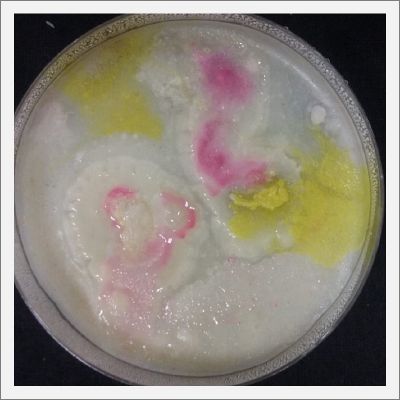
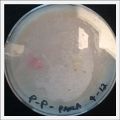
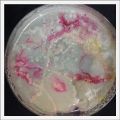
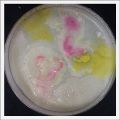
*Day 19: I made an observation under the microscope, the pink color in my samples is a bacteria, a contamination that comes from the first petri dish that I used to extract a clean sample. | *Day 19: I made an observation under the microscope, the pink color in my samples is a bacteria, a contamination that comes from the first petri dish that I used to extract a clean sample. Here can be found more pictures of the contamination. | ||
<gallery> | |||
File:wd39.jpg | |||
File:wd40.jpg | |||
File:wd41.jpg | |||
</gallery> | |||
Revision as of 11:50, 13 December 2018
SELECTED SPECIMENS FURTHER INVESTIGATION
Polycephalum/physarum
- Wikipedia link: https://es.wikipedia.org/wiki/Physarum_polycephalum (general information around this specimen)
- TED: https://www.ted.com/speakers/heather_barnett_1 (a conference opening a window around experiments with this specimen)
- The Slime Mould Collective: http://slimoco.ning.com/
(here can be found experiments around the world around this specimen) really cool :) (personal opinion)
- Video educational video https://www.youtube.com/watch?v=40f7_93NIgA (Simon Garnier - assistant professor in NJIT talks about his studies)
- Slime Molds Remember - but Do they learn? https://www.quantamagazine.org/slime-molds-remember-but-do-they-learn-20180709/?fbclid=IwAR193K1ULzbxSq-BNUijJWsydeAbAHa6-Eq2sXZpdbLl9_ssvLel3Aph_GM " Most importantly, slime molds can be taught new tricks; depending on the species, they may not like caffeine, salt or strong light, but they can learn that no-go areas marked with these are not as bad as they seem, a process known as habituation." (This quote from this article, give me a hint, in order to experiment with salinity in the medium of the Polycephalum). Dussutour make an experiment placing oatmeal at the other side of a gelatine substance made with either caffeine or quinine in order to obligate the Polycephalum to cross, she learned that after the organism get used to a certain substance, they will cross to the food in this case in the same kind of medium that was located in the first time. (the Physarum have "memory" channels). She made also an experiment testing salinity environments, was the specimen adapted himself to this environment, (also she tested with her team if this information could be communicated to other samples that were not "trained" to this kind of environment).
- Video testing intelligence of the specimen https://www.youtube.com/watch?v=czk4xgdhdY4
- Video Polycephalum in technology (computer storage) https://www.youtube.com/watch?v=HKZ2LtfDrmg
- This specimen is found in the forest and is a type of amoeba.
- Grows at a temperature of around 22° - 24°.
- Medium:
- 100ml of distilled water
- 2g of agar
- Leaflets
Aliivibrio Fischeri
- Wikipedia link:
- This specimen is found globally in marine environments.
- Is found in very low quantities in almost all oceans of the world in temperate or subtropical water, lives as a symbiont in the light organ of certain fish and squid species. Grows at temperatures of 24°.
- The bioluminescence of A. fischeri is caused by transcription of the lux operon, which is induced through population-dependent quorum sensing. The population of this specimen needs to reach an optimal level to activate the lux operon and stimulate light production.
- Many people have work with this specimen trying to make their own version of the medium. Common ingredients in most media include Tryptone, yeast extract, glycerol, and roughly 3% NaCl by weight.
- Medium:
- NaCl 30 g
- Glycerol 1 g
- Pepton (Bacxto-peptone) 10 g
- Meat extract 3 g
- Made up with distilled water to 1000 ml
EXPERIMENTATION with Alii Vibrio Fischeri
- Day 1: The extraction of Alii Vibrio Fischeri from a living natural environment (took from an octopus).
- Day 4: I failed to extract the Alii Vibrio Fischeri into the medium, after three days there was no blue glowing as seen in the first day inside the medium that I made. My hypothesis is that the specimen wasn't in the same original conditions from where it comes from, and it couldn´t survive.
CONCLUSIONS: Because I failed in the extraction of the Aliivibrio Fischeri, I will start with the cultivation of Polycephalum/physarum in order to confirm if this specimen can be cultivated in salty environments. According to the previous investigations of Dussutour, it is possible, so I will explore this idea.
EXPERIMENTATION with Polycephalum/physarum
- Day 1: In order to test if this specimen can grow in salty environments I made the following sample. In the right the two first specimens of Polycephalum/physarum are in the medium with; 0.5 gr Agar, 25ml water and 1 flake Leaflets. In the middle the medium is divided in the half, the right half has the previous medium and in the left, the medium is made with; 3% of NaCl (simulating the salinity of the sea), 0.5 gr Agar, 25ml water and 1 flake Leaflet is situated 1mm inside the salty environment. The left sample is made totally with the previous medium (the salty medium simulating the salinity of the sea).
After 5 days the samples of Polycephalum/physarum din't came to live again.
- Day 2: I made a new sample with a new medium. Parallel to the samples that I use for the experiment I started to use this new Medium to grow the specimen (to have live healthy samples to experiment), I started from the formula that Fernanda Caicedo gets to in her previous experiments, and I altered for my special use.
Medium:
- 100 ml of water
- 4 gr of cooked potatoes
- 4 gr of oat flakes
- (mix together)
- 2 gr of agar
- (mix all together and boil)
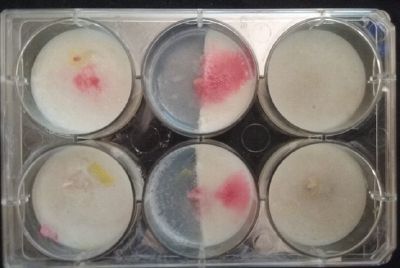
At left a normal medium, at the center a sample half with the normal medium and the other half with 3% of NaCl, and the third sample with just the salty sample.
- Day 3: After one night, the sample in the normal environment is growing normally, the sample in the half mediums moved to the non-salty medium and the one in the salty medium seems that have died.
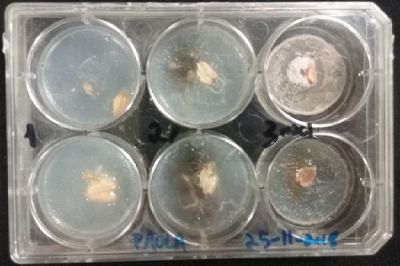
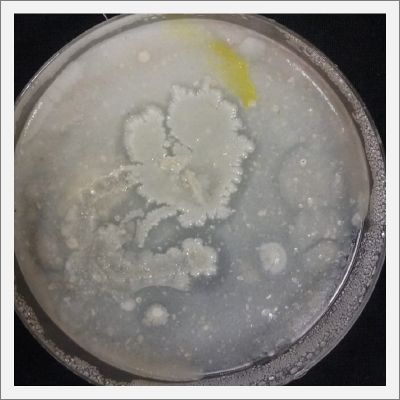
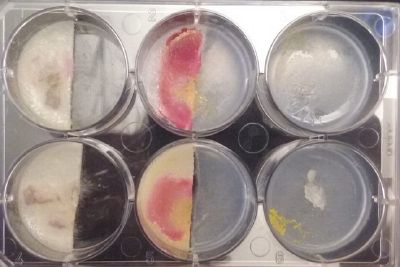
- Day 7: After 10 days the specimen in the normal conditions environment is trying to find new food, is moving because of some contamination inside the Petri dish, the sample with the half environments didn't growth well, and it seems to be some kind of mutation/contamination/reaction of red color, this event also happened with the samples that I'm growing in order to use in these experiments. The specimens in the salty medium died, but one of the samples have the red color.
NOTE: Samples with the pink/red contamination/mutation/reaction. Other samples that I'm growing using the same made medium and the same original Petri dish with the specimen don't have this situation. I don't have an explanation for this fenomena in the moment.
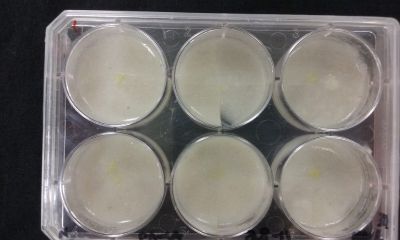
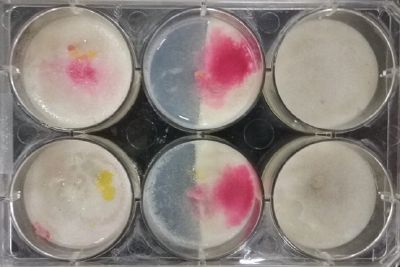
- Day 8: A made some new medium (this time for the salinity medium I just used 2% of salinity concentration) and made a new experiment, this time in the divided medium I used just agar at the left and the salinity 2% concentration medium at left. Two control samples at left with the ideal medium for the growth of the specimen and at the right full concentration of salinity in all the Petri dish.
After one night the specimen in the ideal medium is already growing, the specimen in the middle medium is not moving like the last time (probably because this time the food is concentrated in the salinity medium) and the samples in the salinity medium seem to be dying again.
- Day 10: The living samples are turning pink again, and the samples in the salinity medium seem to be like they are dying again.
- Day 12: Definetly the samples in the salinity medium are death. Ant the contamination/chemical reaction continues in the other samples.
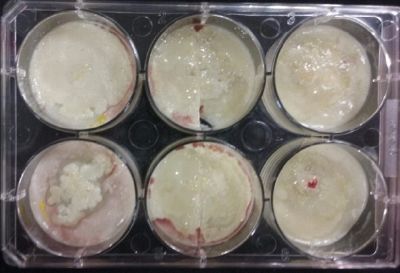
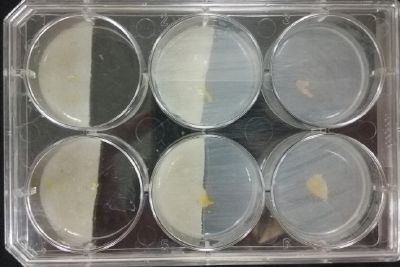
- Day 15: Because the salinity in the medium is to high for the specimens to growth, I reduced the salinity inside the medium into .3% in order to see the reaction of the specimen. The right samples continues to be just a control sample with an ideal medium and the medium in the middle is a medium in between with food in the none salinity medium.
- Day 17: For the ideal medium the specimen is growing, but after a week it acquire some mode, the middle sample move to the food area away from the salinity medium, and the specimen in the salinity medium started to move in order to try to find food. It is tolerating the salinity medium. The specimen in the salinity medium with 0.3% concentration of NaCl is starting to get into hibernation mode, I put some food in order to make it strong before transplanting it into a new medium with higher concentrations of NaCl.
- Day 19: I made an observation under the microscope, the pink color in my samples is a bacteria, a contamination that comes from the first petri dish that I used to extract a clean sample. Here can be found more pictures of the contamination.