Haematococcus pluvialis
Introduction
Haematococcus pluvialis is unicellular freshwater microalga found in different habitats around the world .it belongs to an order of other 7000 different species similar physiological features. The freshwater unicellular biflagellate(having two flagellate) green microalgae H. pluvialis belongs to the class Chlorophyceae, order Volvocales and family Haematococcaseae .It is also known as Haematococcus lacustris or Sphaerella lacustris. Haematococcus was first described by J. Von Flotow in 1844 and later in 1899 Tracy Elliot Hazen extensively presented its biology and life cycle .
Physiology
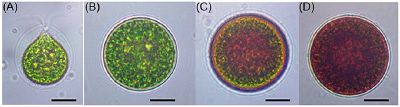
Cellular structure of H. pluvialis is similar to most of other members of volvocalean unicellular green algae. Volvocales are flagellated or pseudociliated green algae known to form circular or spherical colonies. The life cycle of H. pluvialisconsists of four types of distinguishable cellular morphologies: macrozooids (zoospores), microzooids, palmella, and hematocysts (aplanospores) (Hazen, 1899; Elliot, 1934). (Figures 1). Macrozooids (zoospores), microzooids, and palmella stages are usually called “green vegetative phase” (Figures 1A,B). .Hematocysts (aplanospores) are referred as “red nonmotile astaxanthin accumulated encysted phase” of the H. pluvialis life cycle) (Figures 1C,D).. Macrozooids (zoospores) are spherical, ellipsoidal, or pear-shaped cells with two flagella of equal length emerging from anterior end, and a cup-shaped chloroplast with numerous, scattered pyrenoids (Figure 1A). The macrozooid cells are between 8 and 20 μm long with a distinct gelatinous extracellular matrix of variable thickness. Numerous contractile vacuoles are irregularly distributed near the protoplast surface of the cell (Hagen et al., 2002). These flagellated fast-growing vegetative cells predominate under favorable culture conditions in the early vegetative growth stage .
Figure 1. Light microscopic images of H. pluvialis cells in life cycle. (A) Green vegetative motile cell; (B) Green vegetative palmella cell; (C) Astaxanthin accumulating palmella cell in transition to aplanospore; (D) Astaxanthin accumulated aplanospore cell. Scale bar: 10 μm. (https://www.ncbi.nlm.nih.gov/pubmed/27200009 ,Front Plant Sci. 2016)
Reproduction
Macrozooids may divide into 2–32 daughter cells by mitosis (Wayama et al., 2013) (Figures 2A,B). .Under unfavorable environmental or culture conditions, macrozooids start losing flagella, and expand their cell size. They form an amorphous multilayered structure in the inner regions of the extracellular matrix or the primary cell wall as they develop into non-motile “palmella” and become resting vegetative cells (Hagen et al., 2002) (Figure 1B). .With the continued environmental stress (i.e., nutrient deprivation, high light irradiance, high salinity) and cessation of cell division, “palmella” transform into the asexual “aplanospores” At this stage, cells contain two distinct structures, a thick and rigid trilaminar sheath, and secondary cell wall of acetolysis-resistant material. Such cells become resistant to prevailing extreme environmental conditions (Santos and Mesquita, 1984; Boussiba and Vonshak, 1991). Mature aplanospores; accumulate large amounts of secondary carotenoids, particularly astaxanthin, in lipid droplets deposited in the cytoplasm, which results in a characteristic bright red color of these cells (Hagen et al., 2002). Some H. pluvialis strains are reported to be capable of accumulating astaxanthin without forming aplanospores (Brinda et al., 2004). Once environmental or culture conditions return to optimal, red aplanospores germinate to form flagellated zoospores to initiate a new vegetative growth cycle gametocysts. Sexual reproduction is rarely observed in H. pluvialis, and has been largely replaced by vegetative reproduction (Triki et al., 1997).
In some cases, gametogenesis may occur in aplanospores .Such process requires an exposure to extreme adverse conditions (freezing, desiccation, or nutrient starvation) followed by return to favorable culture conditions. During gametogenesis, aplanospore cells can produce up to 64 gametes which are known as microzooids. The microzooids are smaller in size (< 10 μm) compared to the zoospores (20–50 μm), and exhibit high-speed motility after their release from gametocysts. Sexual reproduction is rarely observed in H. pluvialis, and has been largely replaced by vegetative reproduction (Triki et al., 1997).
Habitat
H. pluvialis is commonly found in small temporary freshwater bodies and widely distributed in many habitats worldwide. It occurs primarily in transient water bodies like ephemeral rain pools, artificial pools, natural and man-made ponds, and birdbaths.This microalga can be usually found in temperate regions around the world and has been isolated from Europe, Africa, North America, and Himachal Pradeslv India.It has been also found across diverse environmental and climate conditions. It is well suited for survival under conditions of expeditious and extreme in light, temperature, and salt concentration that would be deleterious to many other microalgae, due to its ability to encyst (become enclosed by thick membrane) in a rapid manner (Proctor, 1957). Commercial Uses Many species of microalga are used as source of nutrient rich food and health promoting compounds as well as feed for fishes.Among the commercially important microalgae, Haematococcus pluvialis is the richest source of natural astaxanthin one of the primary choices of antioxidants. Natural astaxanthin produced by H. pluvialis has significantly superior antioxidant capacity than the synthetic one. Astaxanthin has important applications in the nutraceuticals, cosmetics, food, and aquaculture industries. It is now evident that, astaxanthin can significantly reduce free radicals and oxidative stress and help human body maintain a healthy state. With extraordinary potency and increase in demand, astaxanthin is one of the high-value microalgal products of the future.(https://www.ncbi.nlm.nih.gov/pubmed/27200009 ,Front Plant Sci. 2016)